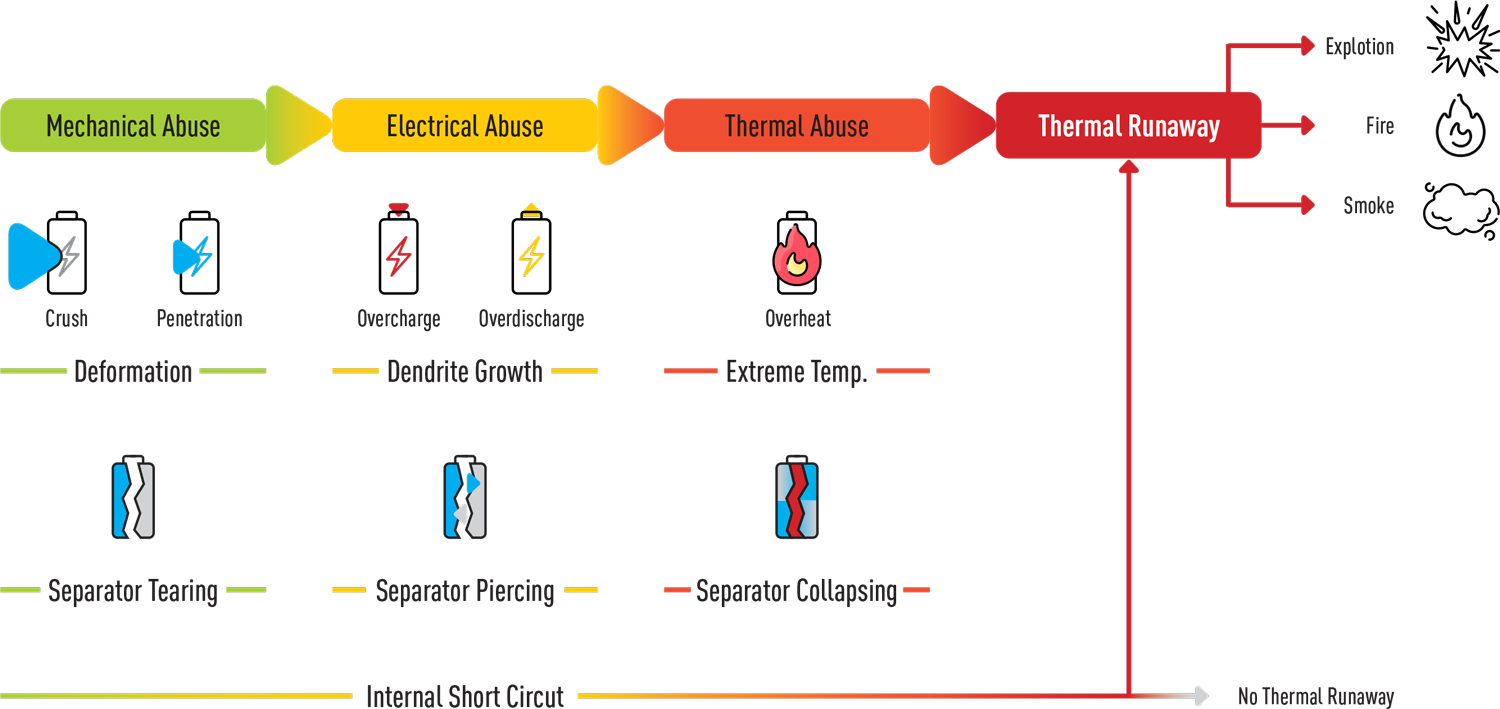
The combustion of a lithium battery always starts with a process known as Thermal Runaway. This internal process develops inside a lithium battery cell as a result of a failure.
This process involves a chemical reaction which generates increasing heat, causing the reaction to accelerate. The released heat causes a chemical chain reaction which releases more heat, and so on. In addition to the chain reaction, a large amount of gas and other toxic and hazardous by-products are released.
The Thermal Runaway process may take a long time and develop slowly until it reaches a critical point where the rate of gas release and temperature increase rises uncontrollably, eventually causes combustion and burning of the raw materials – the metals and gases contained in the cell. A Thermal Runaway may start due to physical damage to the battery, an electric short-circuit or failure, or exposure to aheat source.
The main causes of lithium battery failure:
- External damage to the battery
- Overheating
- Internal short-circuits and failures
- Manufacturing defects